A. Electronic data capture system (Clade-IS)
Conventional data collection for clinical trials traditionally focused on paper-based case report forms (CRF) followed by double data entry into a relational database. Electronic data capture (EDC) systems represent an intriguing alternative, which allow investigators to enter and to review data in real time and to implement on-line data validation checks, thus assuring data quality more effectively at the point of entry.
 CLADE-IS (Clinical Data Warehousing Information System) belongs to the class of the most modern and progressive EDC systems. It comes with EAV data-model of the database (Entity–Attribute–Value model), which allows to switch quickly between various topics and clinical fields where data needs to be collected, verified, analyzed and visualized on-line. The inherent data-access model is robust enough to allow countless configurations of user privileges, roles and data flow. In the default configuration, the following user roles are recognized inside CLADE-IS: (i) investigator, (ii) site manager, (iii) regional coordinator, (iv) data manager, (v) monitor, (vi) administrator.
CLADE-IS (Clinical Data Warehousing Information System) belongs to the class of the most modern and progressive EDC systems. It comes with EAV data-model of the database (Entity–Attribute–Value model), which allows to switch quickly between various topics and clinical fields where data needs to be collected, verified, analyzed and visualized on-line. The inherent data-access model is robust enough to allow countless configurations of user privileges, roles and data flow. In the default configuration, the following user roles are recognized inside CLADE-IS: (i) investigator, (ii) site manager, (iii) regional coordinator, (iv) data manager, (v) monitor, (vi) administrator.
Download
Brief characteristics of Clade-IS – electronic data capture system ( 3.8 MB)
3.8 MB)
With the use of responsive webdesign, CLADE-IS provides its users with an easy and ergonomic interface. Navigation and reading require only a minimum of resizing, panning, and scrolling; a wide range of devices can be used: from desktop computers to tablets and smartphones. CLADE-IS works in the majority of available web browsers; there is no need to install any other software. It is recommended to use only up-to-date browsers running on up-to-date operation systems.
Only authorized users – based on their login and password – can access the interface of CLADE-IS. The registered data is anonymous: for each patient/case, a unique ID is always generated. The communication between CLADE-IS and its users is encrypted with the use of SSL (Secure Sockets Layer).
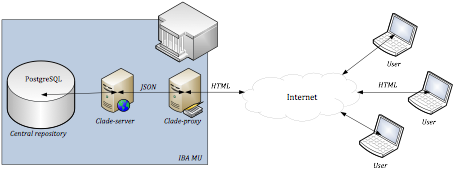
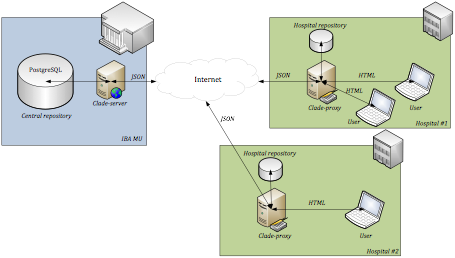
CLADE-IS can be used in two different setups: (i) multicentric data collection, as shown in Figure 1; (ii) ICT infrastructure for clinical data collection and harmonization in the centralized data warehouse, as shown in Figure 2. The latter setup represents an optional way how to collect personal data and store them outside the central repository of the system: the personal data, if collected, are stored in a data warehouse run as part of IT infrastructure of the respective health care facility.
Figure 1: A typical setup of CLADE-IS providing solution for a multicentric data collection. (Click on the image to enlarge it.)
Figure 2: An optional de-centralization of CLADE-IS, which might be used particularly in cases when collection of personal data is necessary: the Clade-proxy component is implemented at each site of data collection, ensuring the filtration of personal data before each transfer of data to the central repository. (Click on the image to enlarge it.)
B. Multicentric data collection (TrialDB2)
Data security within the registry is of key importance, and a special attention must be paid to this issue. Data of individual projects are stored in a database system which was originally based on a modified version of TrialDB system [1–3]. This on-line system has undergone changes in layout and structure, which has made data entry even more comfortable, while security measures have been maintained at the same level as before.
The system has been designed as a robust base for collection of large amount of data in clinical trials and/or clinical registries, is fully customized to the structures of individual projects. The on-line application is accessible to users via the web browser. The security of individual records within the registry is guaranteed via de-identified data collection. The identity of each patient is replaced with a number (ID), which does not allow any backward identification of that person. The unequivocal identification of patient is only known to the attending physician or to an authorised health care professional.
The main advantages of this system involve centralized administration, uniform appearance of forms for data collection in all registries and easy development of new, extending functions.
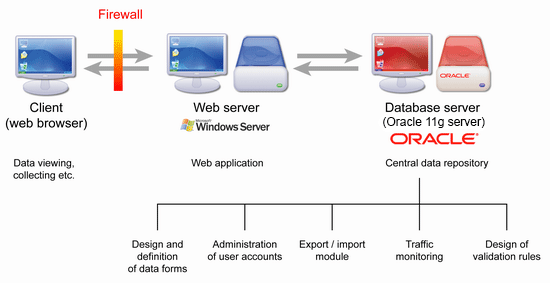
Figure 3: Schematic representation of TrialDB2 – database system used by IBA MU in dozens of projects.
References
- Nadkarni PM, Brandt C, Frawley S, Sayward FG, Einbinder R, Zelterman D, Schacter L, Miller PL. 1998. Managing attribute--value clinical trials data using the ACT/DB client-server database system. J Am Med Inform Assoc 5(2):139-151.
- Nadkarni PM, Brandt CM, Marenco L. 2000. WebEAV: automatic metadata-driven generation of web interfaces to entity-attribute-value databases. J Am Med Inform Assoc 7(4):343-356.
- Nadkarni PM, Marenco L. 2001. Easing the transition between attribute-value databases and conventional databases for scientific data. Proc AMIA Symp:483-487.